When a specimen goes missing, care plans stall and trust suffers. Manual steps and line-of-sight scans leave gaps that are hard to manage across a network. This guide shows hospitals and IDNs how to use RFID specimen tracking to secure the chain of custody, locate items quickly, and standardize processes across sites.
What Hospitals and IDNs Need
- •Complete, searchable chain of custody across inpatient units, clinics, couriers, and core labs
- •Fast recovery when a specimen goes off route
- •Audit-ready records for CAP and Joint Commission
- •Integration with LIS and EHR, without adding work for staff
In many cases, the barcode remains the specimen identifier and print workflow. Passive RFID adds the time and place events you cannot get from line-of-sight scans, along with rapid recovery tools.
How Passive RFID Delivers Custody and Recovery
RFID labels at collection
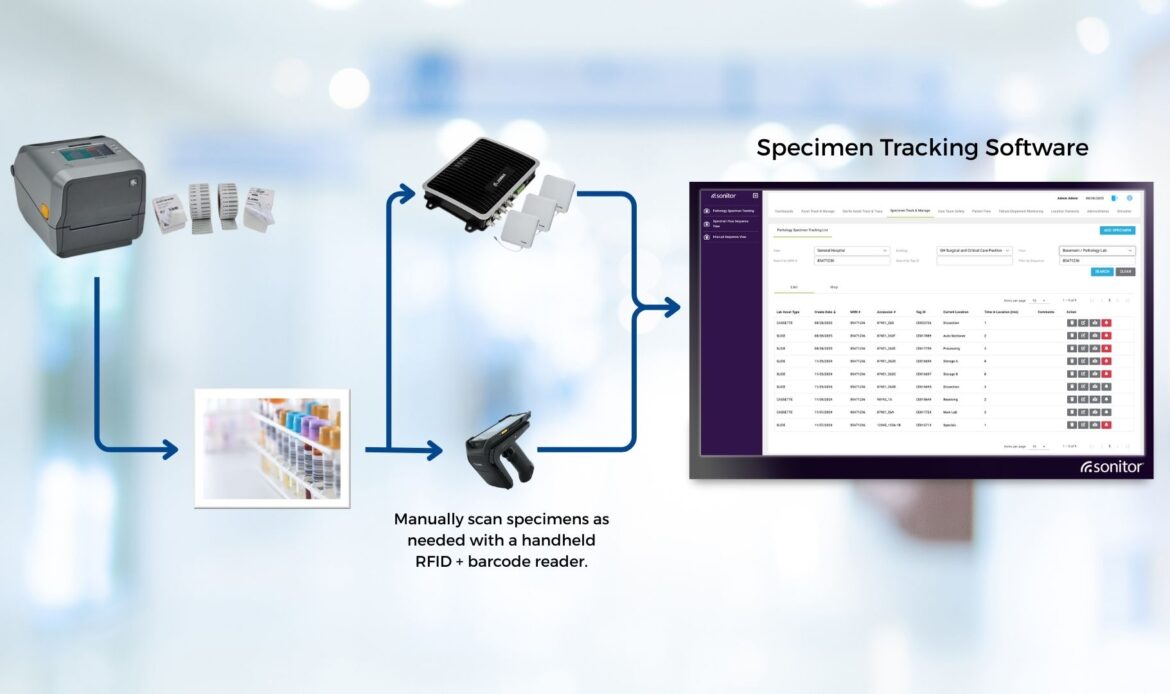
Apply a passive RFID label when you print the specimen label. The tag carries a non-PHI key that links to the LIS order number (accession). Hospitals typically either encode the RFID at print time or use pre-encoded RFID labels and associate them with the LIS order number with a hybrid scanner.
Fixed readers at handoffs
Readers and antennas are typically mounted overhead and placed at defined checkpoints such as unit exits, pneumatic-tube entry/exit, lab receiving thresholds, refrigerators and freezers, and processing benches. These automate movement events without extra steps for staff.
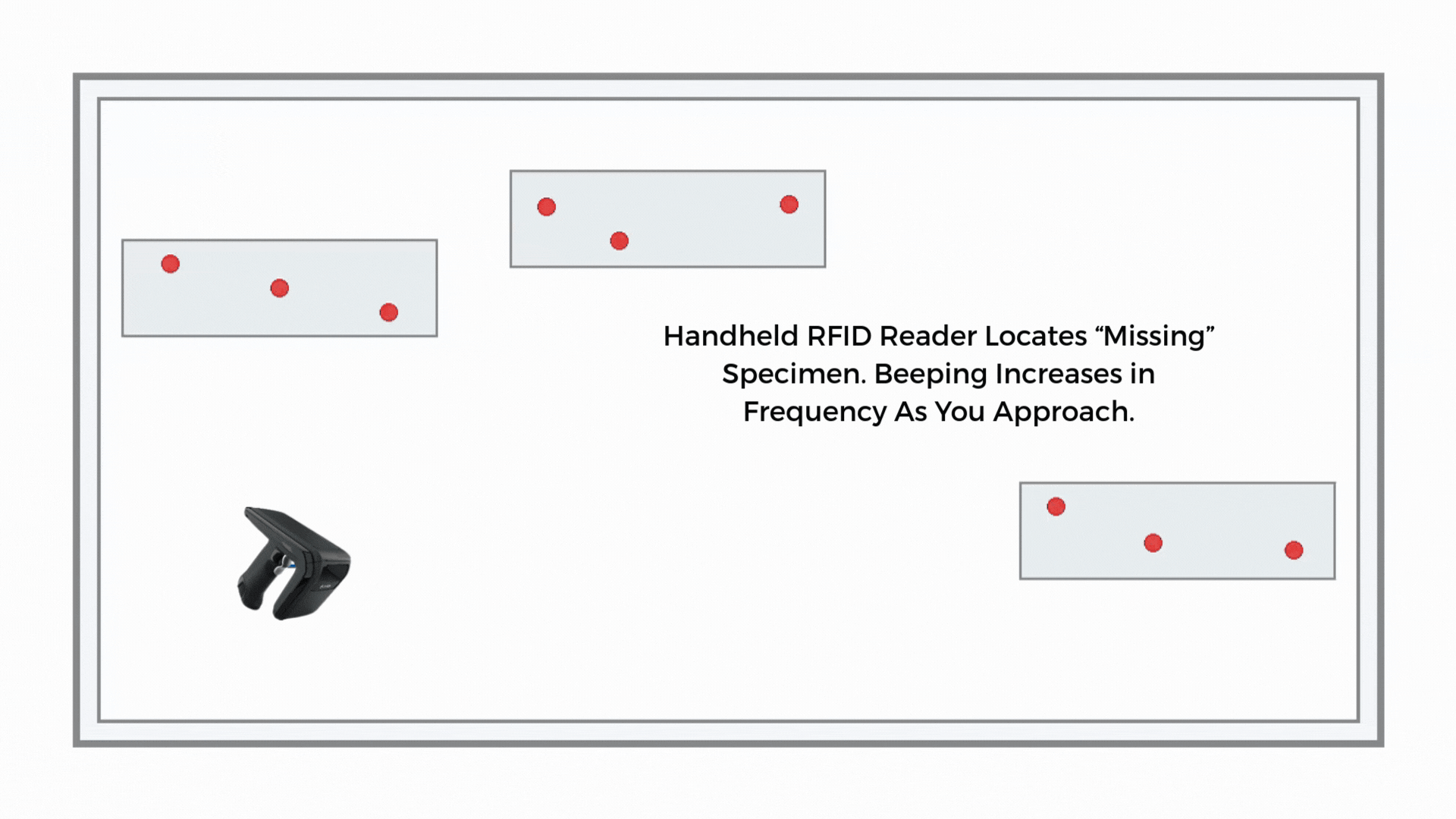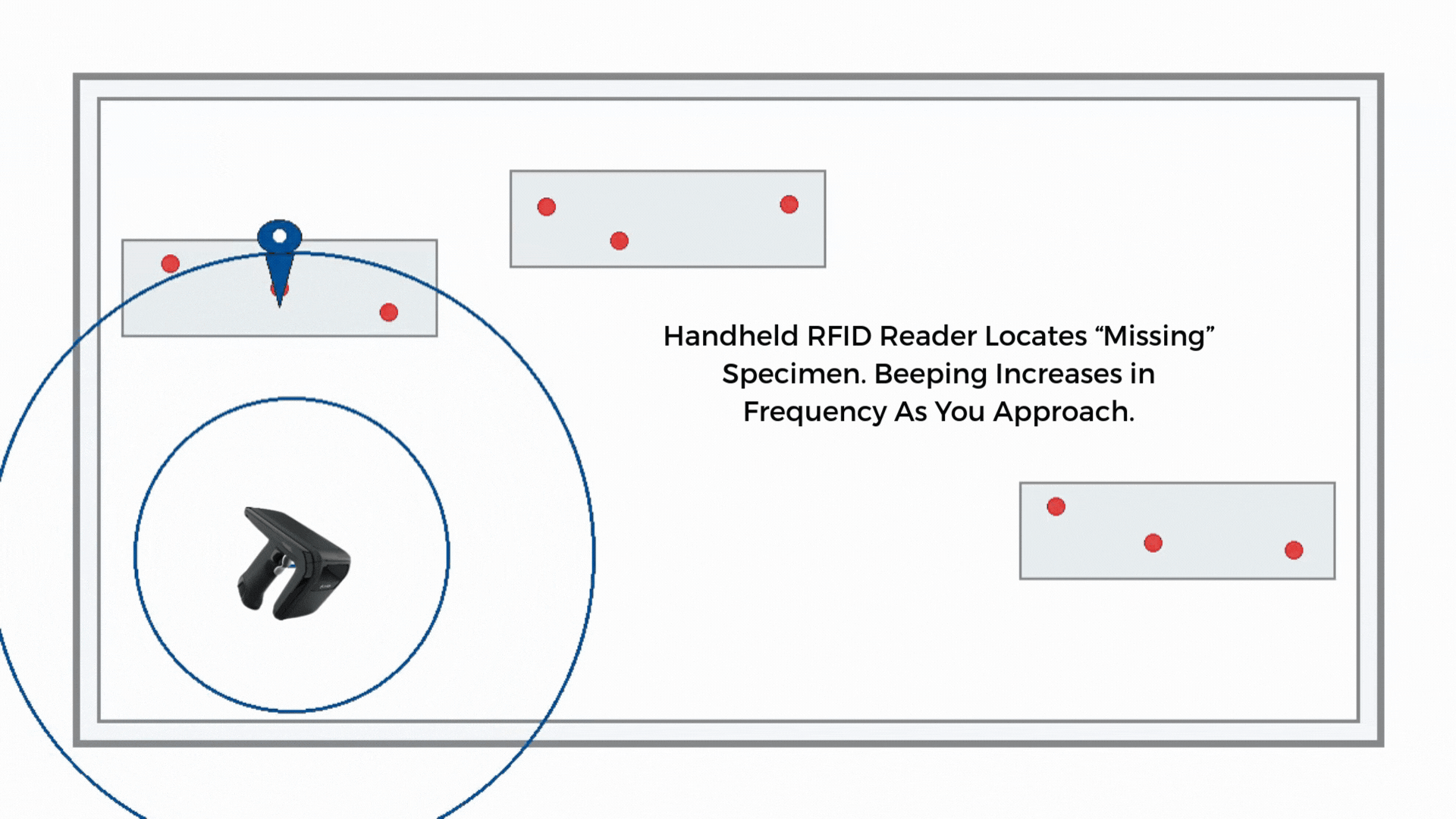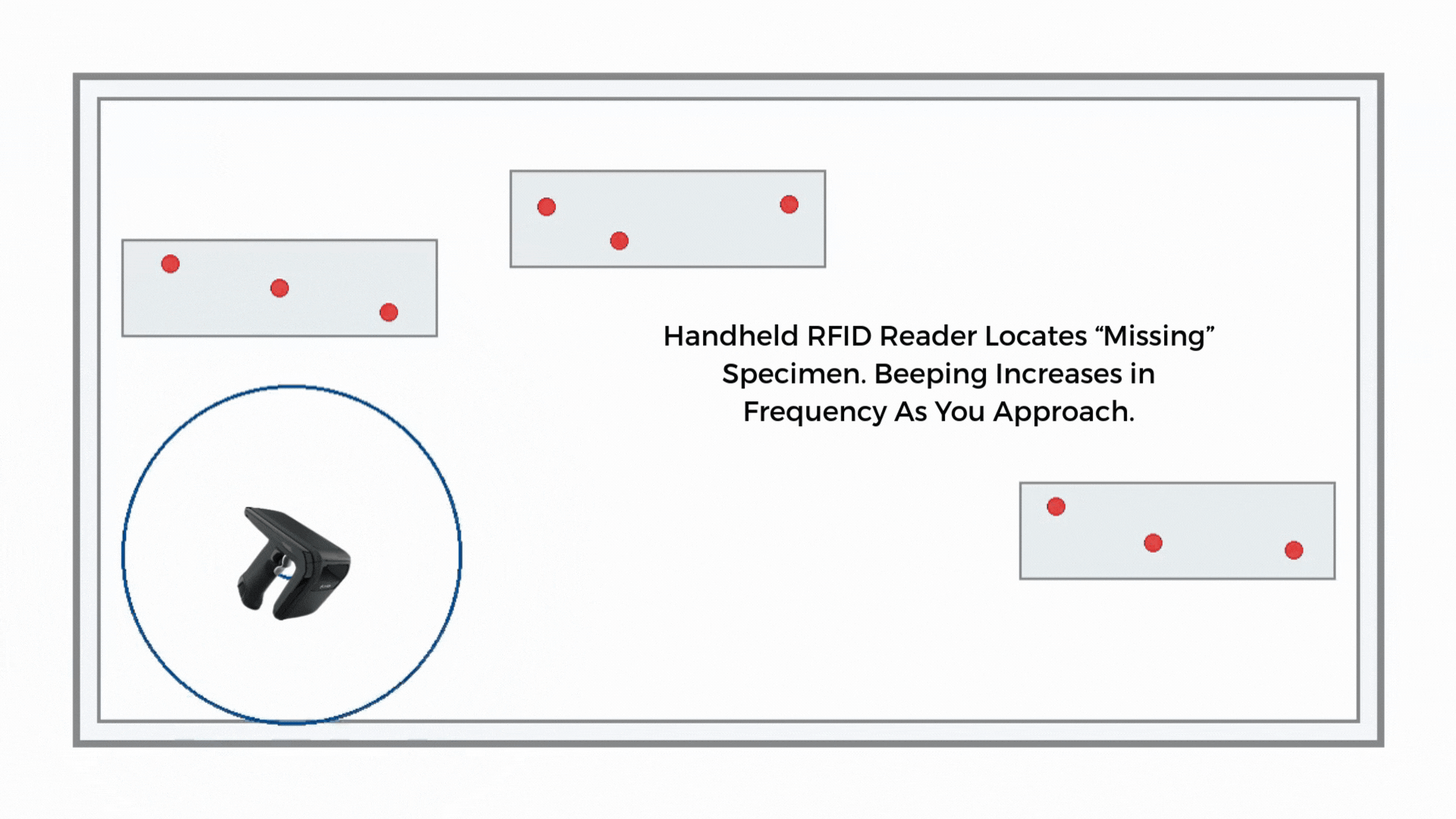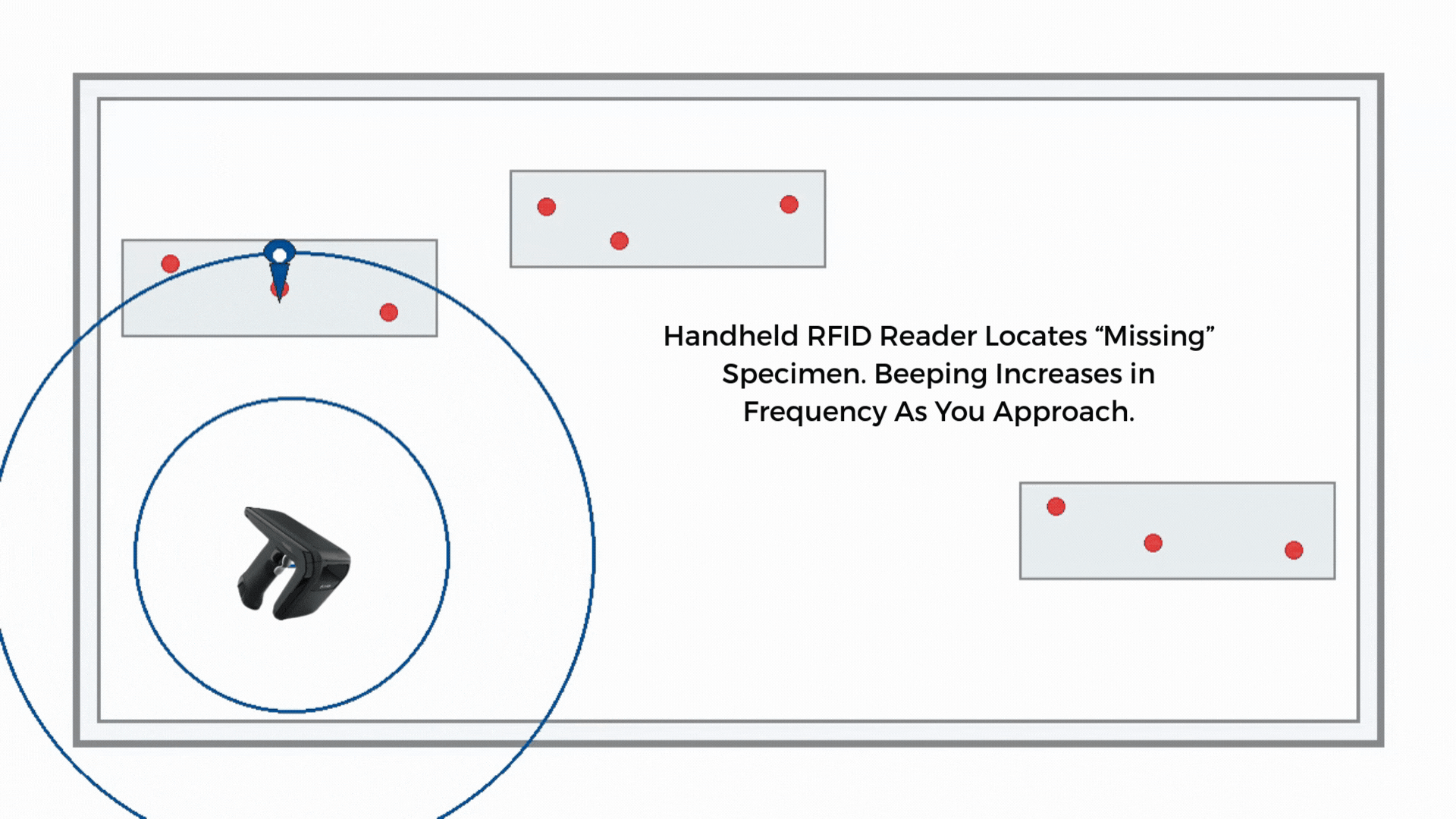
Handheld recovery
If a sample is misplaced, a handheld RFID reader guides staff by signal strength. Search time drops from minutes to seconds in many environments, and the last-seen zone narrows the hunt.
See RFID specimen tracking in action.
Contact us to schedule an in-person or remote demo for your hospital or IDN.
Cover The Transport Leg with Automated Checkpoints
RFID portals capture custody at collection and at receiving. To keep custody continuous while a specimen is moving, combine fixed readers at key thresholds with simple handheld steps where portals are not practical.

Pick up checkout at the unit:
Use a handheld RFID reader to associate each specimen’s RFID tag with a bag or cooler ID and record the LIS order numbers inside. In high-volume units, a short-range bench reader can automate this association as items are packed.
Automatic departure event:
Install a fixed RFID reader at the unit exit or dispatch hub. When the bag passes through, the system records a departure event and starts an expected arrival timer for the route.
Automated arrival at receiving:
Place a fixed reader in the receiving threshold. As the bag crosses, the system logs arrival, reads the specimen tags inside, and reconciles which LIS orders were expected versus received. Any missing or unexpected items are flagged immediately.
Exception handling and recovery:
If the route timer expires or reconciliation shows a gap, staff use a handheld to perform a proximity search along the courier path, storage areas, or staging racks. The handheld guides the search by signal strength to shorten the time to find.
Cold chain, when required:
For refrigerated specimens, place a simple temperature logger inside the cooler. On arrival, the system records a pass or fail result alongside the custody events.
Pneumatic tube checkpoints:
Place read zones at tube entry and exit to log custody as specimens move between buildings.
Start small. Many hospitals begin with a receiving portal and a handheld checkout at the unit. As volume grows, add unit exit portals on the busiest routes to remove manual steps and tighten timing.
Searching for more concise and shareable information?
Download our Specimen Tracking flyer.
RFID Specimen Tracking Operating Blueprint for IDNs
Focus on network standards, exception control, and scale.
1) Standardize the RFID specimen tracking model
•Tag and label policy: approved RFID labels by container and temperature range, link each tag to the LIS order number.
•Read-zone patterns: unit checkout bench or handheld, unit exit portal on high volume routes, receiving portal, cold storage spot reads.
•Bag and cooler IDs: consistent IDs for courier containers with simple association to included LIS orders.
•Privacy and retention: non-PHI on tags, event retention rules, audit log access.
2) Control exceptions in hospital specimen tracking
•Missed arrival: timer starts at unit exit. If receiving does not see the bag within the route window, alert the team.
•Partial receipt: receiving reconciliation flags LIS orders expected but not seen. Open a task to locate.
•Unreadable tag or damaged label: fall back to barcode, re-tag, and capture a correction event.
•Cold chain fail: temperature logger result posts pass or fail beside the custody trail.
3) Roles and accountability for RFID specimen tracking
•Clinical operations: nursing and phlebotomy pack and associate to bags, request pickup.
•Courier services: move through exit portals, confirm pickup if handheld is used, and deliver through the receiving portal.
•Lab receiving: reconcile expected versus received, handle exceptions, re-tag when needed.
•Hospital IT and InfoSec: provide network access, device whitelisting, identity and SSO, endpoint management, change control, and security validation.
•Implementation partners: manage reader and antenna configuration, middleware, LIS and EHR interfaces, dashboards, and alert rules under a support agreement. Provide training and Tier 2 and Tier 3 support, monitor read rates and portal health, perform preventive maintenance, and handle releases.
•Technology vendors: supply hardware, firmware, and application updates, warranty service, and Tier 3 escalation through the implementation partners.
•Quality and compliance: review exception trends, audit trails, and KPI performance, and prepare evidence for CAP and Joint Commission.
•Support flow: Hospital service desk handles Tier 1. Implementation partners handle Tier 2 and Tier 3 under a support agreement.
4) Integration pattern for RFID specimen tracking and lab asset tracking
•Events to LIS: write time and place events tied to the LIS order number. Tags carry a non-PHI key.
•Operational visibility: dashboards show last seen zone, route timers, receiving reconciliation, and cold chain status where required. Alert on exceptions only.
•Security and governance: SSO, role-based access, audit logs, and device network segmentation with standard change control.
•Interfaces and reliability: use the hospital interface engine or approved APIs. Buffer and replay during outages. Design events to avoid duplicates.
•Retention: align custody event retention with quality and accreditation requirements.
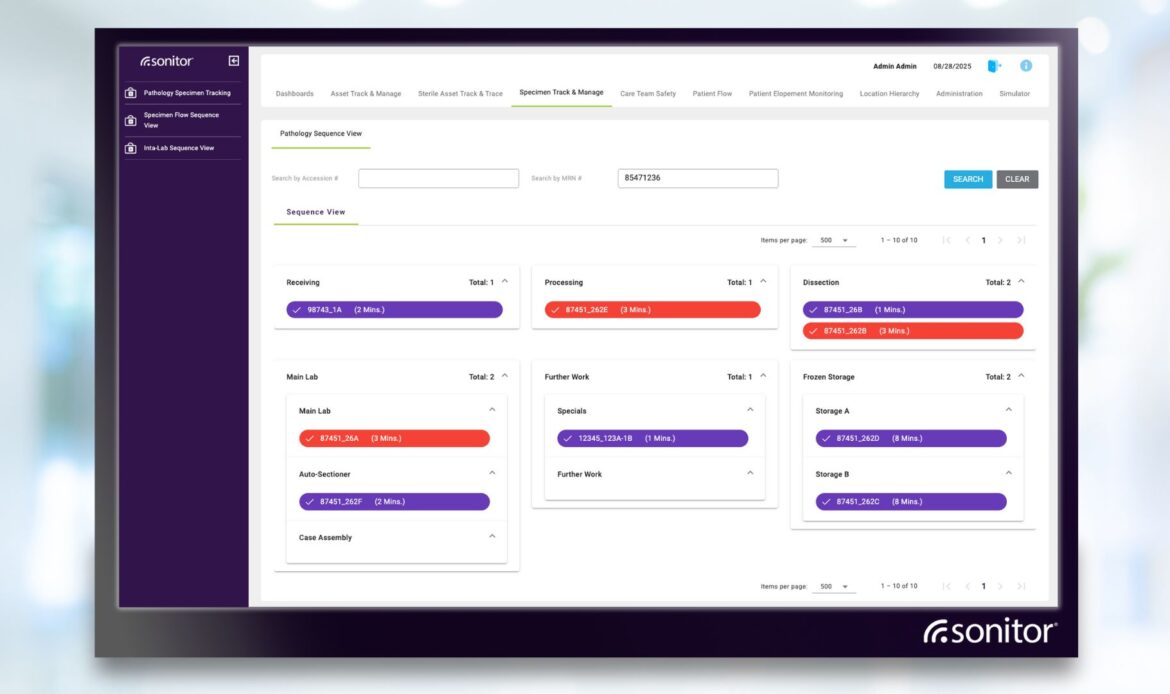
•Workflow views: a list view supports search and filter by location, specimen type, date, LIS order number, and sequence. A sequence view shows progress by state with time-in-stage thresholds and color-coded alerts. Location updates can be automatic from RFID infrastructure or added manually from handheld RFID or barcode scans.
5) Phased rollout of RFID specimen tracking
Phase 1: receiving portal plus handheld checkout on one high-volume route
Phase 2: add unit exit portals on the busiest floors
Phase 3: extend to the courier hub and cold storage zones
Phase 4: onboard ambulatory clinics and send-outs with the same patterns
Phase 5: standardize across hospitals with super user model and central governance.
6) Network KPIs for hospital specimen tracking
| Lost or delayed specimens per 10,000 LIS orders. | Cold chain failures per 1,000 cooled shipments. |
| Average time to locate a missing item. | Re-collection rate and cost avoidance. |
| Collection to receipt turnaround by priority class. | Percentage of specimens compliant within time-in-stage thresholds. |
| Percentage of LIS orders with complete custody events. |
What good looks like for hospital specimen tracking at IDN scale
•Find time: median time to locate a misplaced specimen under 5 minutes
•Custody completeness: 95 percent or higher of LIS orders show a full trail from collection to receipt, with 99 percent on priority classes
•Receiving reconciliation: 90 percent of expected items auto-matched within 1 minute of arrival, 100 percent of exceptions reviewed the same shift
•Turnaround: collection to receipt meets target by priority class, with clear thresholds for STAT and routine
•Re-collections: measurable reduction within the first 90 days
•Cold chain: fewer than 3 excursions per 1,000 cooled shipments, where applicable
•Reliability: portal uptime over 99.5 percent and healthy interface queues
FAQ for RFID specimen tracking in hospitals and IDNs
Q. Will RFID work with liquids and cold storage?
A. Yes, with the right label and placement. Validate with a detailed site survey.
Q. Does RFID replace barcode?
A. No. Barcode remains the identifier. RFID adds automated custody and location.
Q. Will staff have many new steps?
A. Fixed readers automate most events. Handheld steps focus on checkout or exception recovery.
Q. How long does a pilot take?
A. Many pilots complete in six to eight weeks after a paid site survey and design.
Q. Can this run in our environment?
A. Yes. Deployments can be cloud-hosted or on-premises, aligned with hospital IT policies.
Q. Can staff add a location without a portal read?
A. Yes. Manual location updates from a handheld RFID scan or a barcode scan can write a location event into the record.
See RFID specimen tracking in action
Schedule an in-person or remote demo for your hospital or IDN. Call us at (425) 438-2533 or contact us here. Curious about more use cases for healthcare automation? Download our Solutions Guide, “Transforming Healthcare Operations: Smart Automation for Hospitals and Patient Care.”
